December 03, 2019

Ketcham Professor of Biomedical, Biological and Chemical Engineering Elizabeth Loboa says her research warns of the potential side effects of some osteoporosis treatments because they might damage the neighboring cartilage.
It is well-known that a lack of physical activity can cause bone loss or fractures in elderly people, bedridden patients or even sedentary youth. That’s because bone mass is regulated, in part, by mechanical stimuli. Similar to muscles, bones add mass when they are mechanically loaded via physical activity but will lose mass if immobilized or when a person spends time at low or zero gravity, such as an astronaut. New research from Ketcham Professor of Biomedical, Biological and Chemical Engineering Elizabeth Loboa, dean of MU Engineering, along with her former doctoral student Rachel Nordberg and others, finds that similar processes occur with cartilage.
“A lot of what I do in my group is focus on the effects of mechanical stimuli on biological processes,” Loboa said. “Every organ and tissue in our bodies is subjected to mechanical loads, so a lot of work in my lab looks at how we can optimize and better understand how mechanical loading can either help to regenerate new tissues or help to maintain bone and cartilage health.”
The new paper published in the journal Plos One notes that cartilage also is highly responsive to mechanical stimuli, but the mechanisms by which the cells located in cartilage—chondrocytes—respond to stimuli is not clearly understood. Chondrocytes are the primary cells located in cartilage and they produce and maintain the cartilage matrix – which can be thought of as cells swimming in a lake.
Loboa says one goal of the study was to determine how certain receptors respond to mechanical stimulation. Receptors are proteins that receive and transduce signals that may be integrated into biological systems. These signals are usually chemical messengers that bind to a receptor and cause some form of cellular response. In particular, her team looked at how low-density lipoprotein receptor-related proteins (LRPs) are regulated in simulated microgravity, such as the environment an astronaut would experience, and cyclic hydrostatic pressure, or what we typically experience by just walking. Loboa’s team investigated the potential role of LRP 4, 5 and 6 in response to mechanical loading in order to better understand how to maintain cartilage health.
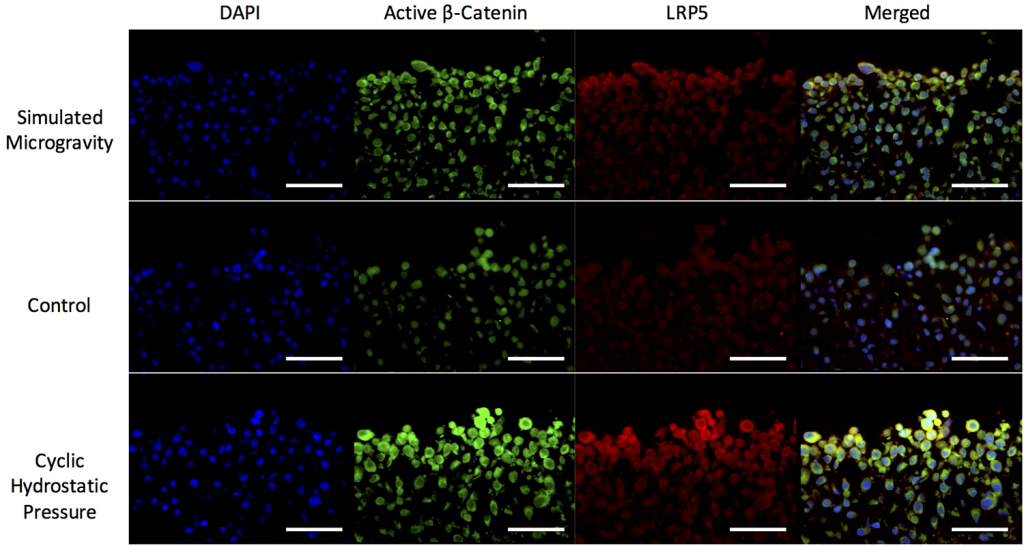
Immunofluorescence of LRP5 and β-catenin in response to mechanical stimuli. LRP5 and active β-catenin after 14 days of culture in simulated microgravity, unstimulated control (6-well plate, no loading) or cyclic hydrostatic pressure.
“We’re focusing on these specific receptors because they are known to be critical receptors in musculoskeletal biology, and this is the first time we’ve evaluated how they respond to mechanical stimulation,” Loboa said.
She says LRP 5 was the most interesting, and previous studies have found it to be associated with osteoarthritic cartilage destruction. Her team then did a deep dive on LRP 5, testing it in vitro (in the culture dish) and in vivo (in mouse models). The next step would be large animal testing, and Loboa says the findings may lead to some kind of pharmaceutical agent.
Her team then tested different levels of sclerostin on LRP 4/5/6. Sclerostin is a small protein produced in osteocytes, which are a type of bone cell. The main function of sclerostin is to stop bone formation, which is necessary to ensure that bones are of the correct shape, size, and density. She says LRP 4 had the largest dose-dependent response.
“Sclerostin has been found to be unfavorable to bone but we think it may be favorable to cartilage and may be acting as a defense mechanism to prevent excessive cartilage degradation,” Loboa said. “Currently, what some researchers are looking at for osteoporosis treatment is to diminish sclerostin, because with osteoporosis you want to help the bone. It’s the same with astronauts—some researchers recommend diminishing sclerostin because it is unfavorable to bone, but we think that when you diminish sclerostin it may hurt the cartilage.”
Loboa says her research is warning of the potential side effects of some of the current sclerostin targeting osteoporosis treatments because they might be damaging the neighboring cartilage, which has implications for aging, arthritis and osteoporosis.
“We need to have a better understanding of mechanical loading that can be used to help modulate both the negative and positive effects, so maybe we can optimize some of the mechanical loading to continue to help the cartilage if someone is being treated for osteoporosis,” Loboa said. “We may need a different approach of how we modulate it pharmaceutically to ensure we are promoting both bone and cartilage health.”

